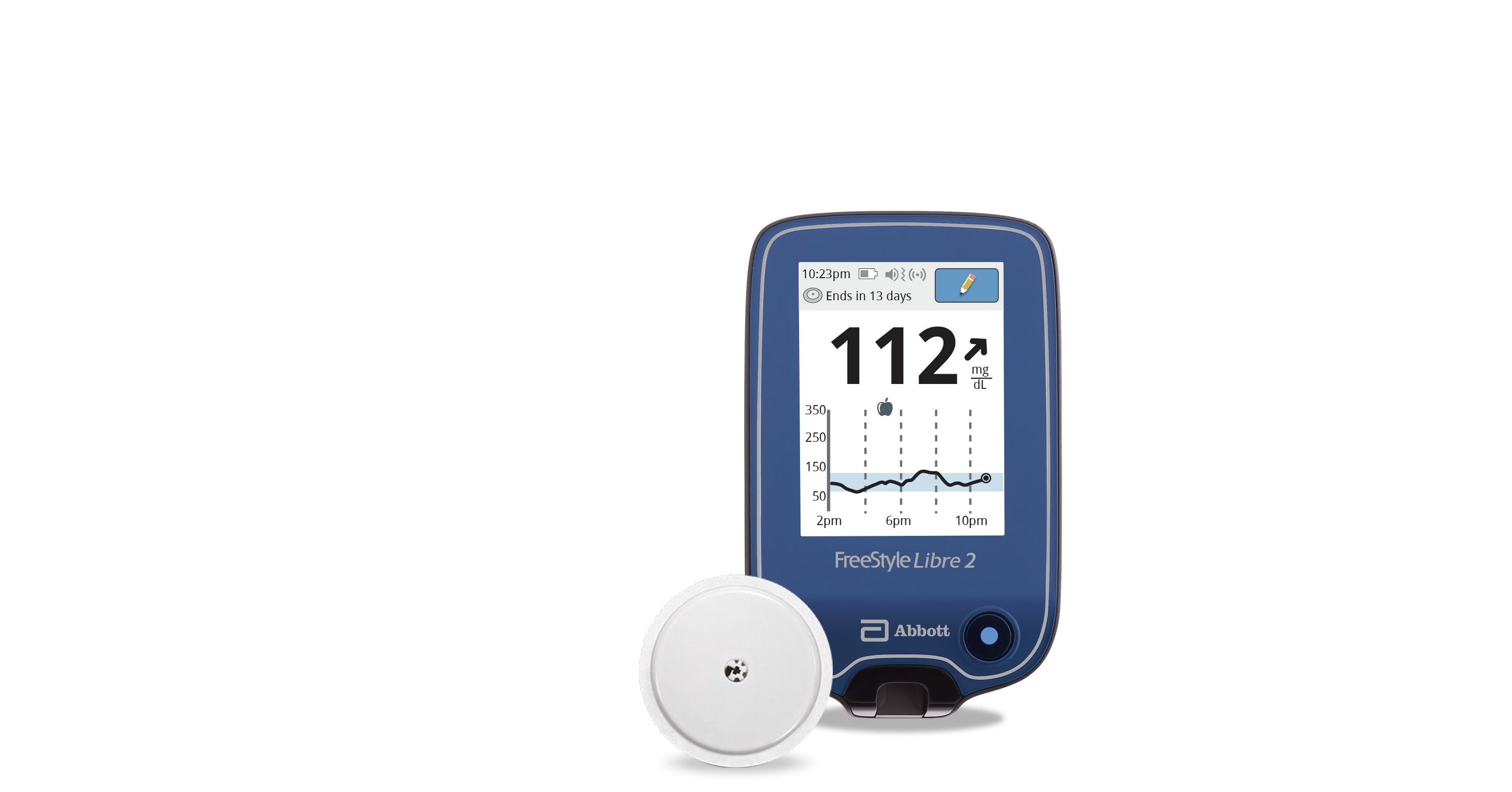
Voluntary Urgent Medical Device Correction Notice for FreeStyle Libre
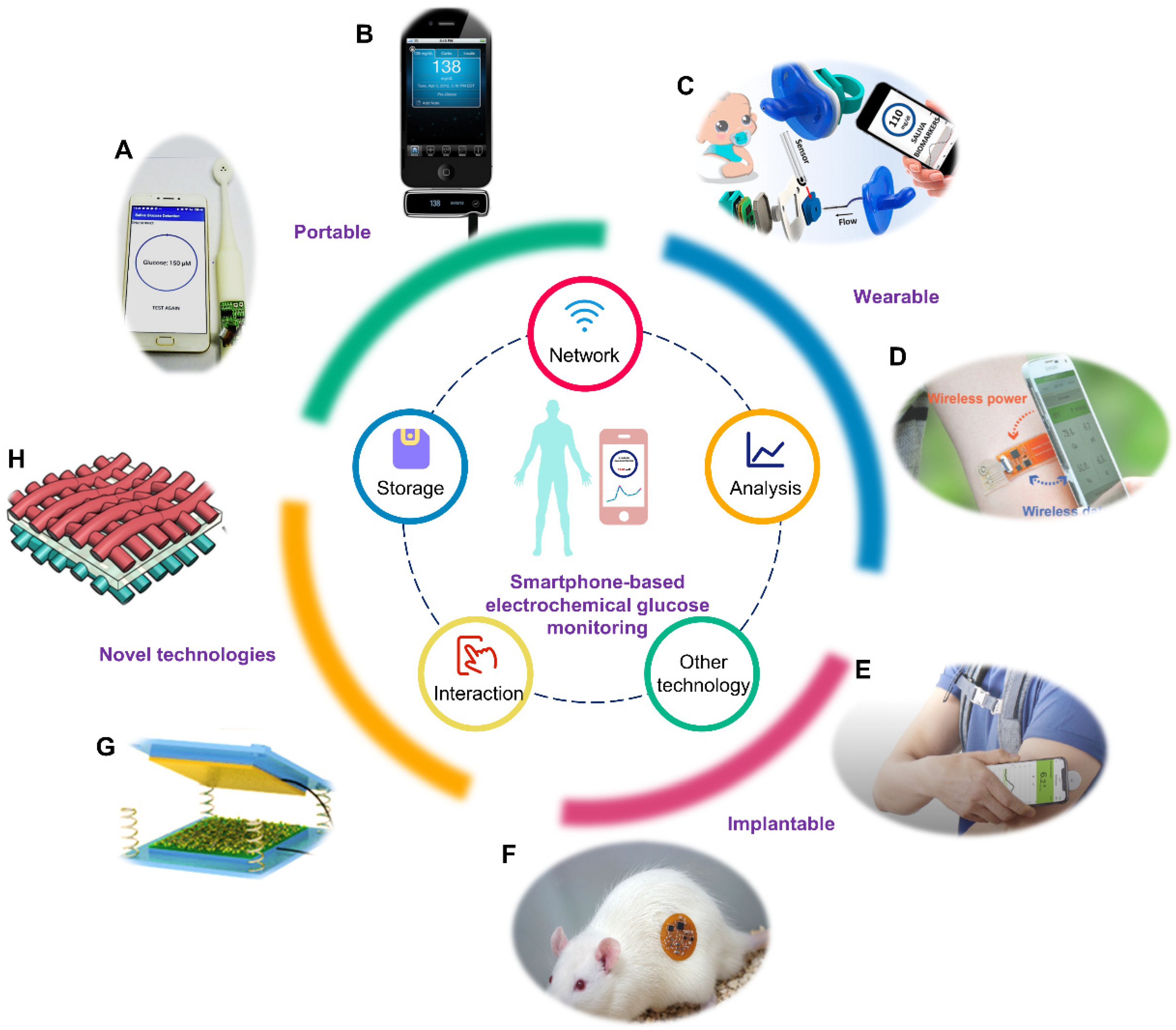
Sensors, Free Full-Text

FreeStyle Libre Now Available in Major US Pharmacies

Budget Impact Analysis of the FreeStyle Libre Flash Continuous Glucose Monitoring System® in Patients with Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus with Multiple Daily Insulin Injections in Argentina

In the News.. Medtronic safety alert, Omnipod 5 in Europe, T1D Index launched and more! - Diabetes Connections

Alere Triage ® TOX Drug Screen Voluntary Recall Letter 6/11/2012

Diabetes in America with Aaron Neinstein, MD, Rita Rastogi Kalyani, MD, MHS & Jennifer Raymond, MD, MCR - The Washington Post

Stryker Lifepak 15 Urgent Medical Device Safety Notice and Correction — MBEMSC
Drug Recalls

FDA Classifies Abbott's Freestyle Libre Readers Recall as the Most Serious Type
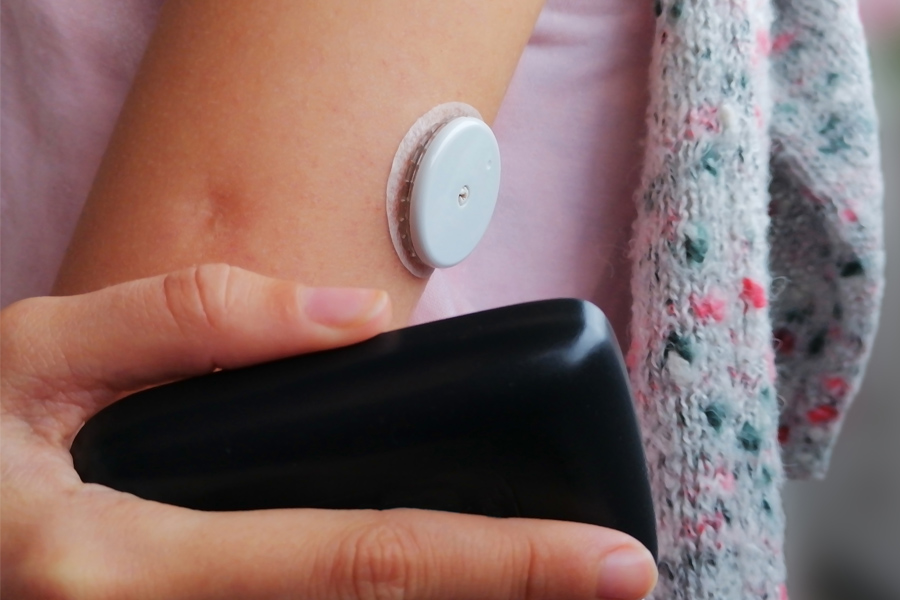
Diabetes Products Shield HealthCare

Freestyle Libre Recall Issued For 4.2M Glucose Monitors After Reports of Fires, Extreme Heat
Voluntary Urgent Medical Device Correction Notice for FreeStyle Libre

April 2023 Central Alabama by Alabama Living - Issuu

Medical Design & Outsourcing – SEPTEMBER 2023 by WTWH Media LLC - Issuu